News
Sign up for recent trade news that can affect your business:

The federal government has announced $50 million to help farmers, fish harvesters, and all food production and processing employers put in place the measures necessary to follow the mandatory 14-day isolation period required of all workers arriving from abroad.
In many regions across Canada, producing a variety of quality foods to be sold at affordable prices relies on the contributions of experienced temporary foreign workers right from planting season to harvest.
Key points:
“This is an important reason why the Government of Canada granted an exemption for temporary foreign workers from travel restrictions to Canada, along with other foreigners with student and work visas, provided they adhere to a strict 14-day isolation protocol upon arrival,” the statement said.
The government stated that those who do not comply with the Quarantine Act or the isolation protocol will face severe fines and sanctions. Under proposed changes, employers could also face significant penalties, and a possible ban from the program, if they prevent adherence to the self-isolation order.
In addition to the responsibility of paying the workers for the two weeks during which time they cannot work, many employers are also responsible for providing workers with transportation and accommodations, as well as access to food and basic supplies needed to meet all of the conditions imposed by public health authorities.
The federal government is providing support of $1,500 for each temporary foreign worker, to employers or those working with them to ensure requirements are fully met. The funding is conditional on employers not being found in violation of the mandatory 14-day isolation protocols or any other public health order. The program will be available as long as the Quarantine Act is in force and the isolation protocol is followed.

The International Chamber of Commerce (ICC) and the World Customs Organization (WCO) have issued a joint statement calling for increased action on customs and trade facilitation to ensure an effective response to the COVID-19 pandemic.
In a joint statement, ICC Secretary General John W.H. Denton AO and WCO Secretary General Kunio Mikuriya say effective trade facilitation – based on international standards – will play a central role in enabling business continuity and renewed economic growth.
The statement calls for a coordinated customs response to the COVID-19 crisis – including through active participation in multilateral efforts and an open dialogue with neighbouring countries.
“We call on Customs administrations and other government agencies to keep trade flowing by maintaining the continuity of the international supply chain and simplifying and facilitating the Customs processes for essential medical equipment, medicines and food supplies – as well as key support personnel – so as to ensure an effective response to the pandemic and to protect lives throughout the world,” the leaders state.
Click here to read the full statement.
ICC and the WCO have also agreed to coordinate efforts in response to COVID-19 and are partnering to explore potential opportunities aiming to keep trade flowing worldwide and to support strong recovery of the global economy.
To support its members and relevant stakeholders, the WCO has created a dedicated section on its website (view here) and has included several existing and newly developed tools to aid in the facilitation of supply chain integrity within the context of the COVID-19 pandemic.
FEMA has offered more details on its plans for exemptions to a temporary rule banning some medical supplies from being shipped overseas, which lawyers for U.S. and foreign companies said provided much needed clarification for exporters.
President Trump, in an April 3 executive order, invoked the Defense Production Act to stop the export of medical products being used to combat COVID-19 that are in short supply. The following week, FEMA published a temporary rule that said companies must receive the agency’s “explicit approval” before exporting certain medical equipment. The export restriction took effect April 10 and is set to expire in mid-August, according to FEMA.
U.S. Customs and Border Protection has since provided informal guidance on the types of shipments that will be exempted from the export restriction.
In an April 21 Federal Register notice, FEMA codified and tailored the exemptions, which provide a path for some exporters to make sales of medical products not in high demand in the U.S.
The exemptions are as follows:

The Commercial Customs Operations Advisory Committee, which advises the heads of the Homeland Security and Treasury departments on CBP operations, has recommended delaying USMCA’s (also referred to as CUSMA) entry into force until next year. The working group was launched in March under COAC to help solicit guidance from the private sector on the deal’s implementation.
“COAC recommends that CBP, the USTR and its USMCA partners should delay USMCA’s entry into force until no earlier than January 1, 2021 and provide a transition or implementation period for the year where NAFTA qualifying goods with appropriate certificates of origin will be considered to comply under the USMCA,” the advisory panel said in a presentation delivered at the meeting, according to Brenda Barnes, a member of the USMCA working group.
Governments had originally hoped for the agreement to go into affect as early as June 1, however, are now facing pressure from the auto industry, lawmakers, and others to push back of the date as companies attempt to grapple with the impact of the COVID-19 pandemic. Companies are saying that they need more time to comply with USMCA’s new rules of origin.
USMCA will have numerous impacts on import and export activity, including changes to low value shipments, requiring a formal certificate, and de-minimis. Click here to read more about those changes.
Despite recommendations from COAC, John Leonard, Executive Director of Trade and Policy Programs in CBP’s Office of Trade, stated that CBP was still tracking for entry into force on June 1, adding that any change would be announced by USTR.
The advisory group said that should USMCA “enter into force as scheduled, at the very least, CBP and its USMCA partners should grant enforcement discretion by way of an informed compliance period until the trade has had reasonable time to implement each administration’s regulatory and automation requirements.”

In response to the COVID-19 pandemic, the CBSA is temporarily reducing service hours at a total of 27 Canadian land border locations. The temporary hour adjustments came into effect as of April 15, 2020 at 11:59 pm EDT and will remain in effect until the expiration date of the Order in Council made under the Quarantine Act prohibiting entry into Canada from the United States.

CBSA Vice-Presidents Lisa Anawati and Peter Hill have announced that the CARM solution will not deploy in Summer 2021. Related consultation and broader engagement also have been postponed indefinitely. An update will be provided by CBSA before the end of May regarding consultation plans.
CARM, a multi-year initiative, is an online portal which will give the trade community access to border services 24 hours a day, seven days a week.

Prime Minister Justin Trudeau says Canada and the United States have struck a deal to extend current border restrictions between the two countries by an additional 30 days, as the U.S. moves to exempt Canada and Mexico from export restrictions on PPE.
The border was originally closed March 21, restricting non-essential travel between Canada and the U.S. Over the past month, it remained open for trade and commerce, with exemptions also granted for emergency response and public health purposes.
The decision comes as the U.S. is set to ease restrictions on exporting protective equipment through a number of new exemptions, one of which allows supplies to be shipped to Canada and Mexico.

U.S. Customs and Border Protection has launched a new resource on its website dealing specifically trade-related updates and official messaging about the impacts of COVID-19.
Information found on the new website includes Federal Register Notices, and Cargo Systems Messaging Service communications related to COVID-19, as well as updates and announcements in trade programs and cargo security. Additionally, as many other departments and agencies are involved with facilitating international trade, the links to the COVID-19 response websites of partner government entities will also be included.
Click here to visit the new U.S. CBP COVID-19 website.
CBP also announced that the agency’s Pharmaceuticals, Health and Chemical Center of Excellence and Expertise has established a new COVID-19 Cargo Resolution Team (CCRT) tasked with coordinating inquiries about the import of medical supplies and personal protective equipment.
The CCRT can be contacted by email at covid19_relief_imports@cbp.dhs.gov.

Due to the COVID-19 pandemic, the Canadian Food Inspection Agency (CFIA) has temporarily suspended some of its low-risk activities.
Low-risk CFIA activities are those that do not immediately impact the production of safe food for Canadians.
Effective immediately, the CFIA is providing flexibility for certain labelling requirements for foodservice packaged products that have no impact on food safety, as detailed below.
Foodservice products are those used by hotels, restaurants and institutions.
This will help to support the economy, alleviate supply disruptions in Canadian grocery stores, and avoid food waste.
Areas of flexibility include:
The notice applies to foodservice food that has already been packaged and labelled. It also applies to foodservice food that is packaged and labelled within 90 days of the publication of this notice. After this time, this measure no longer has effect.
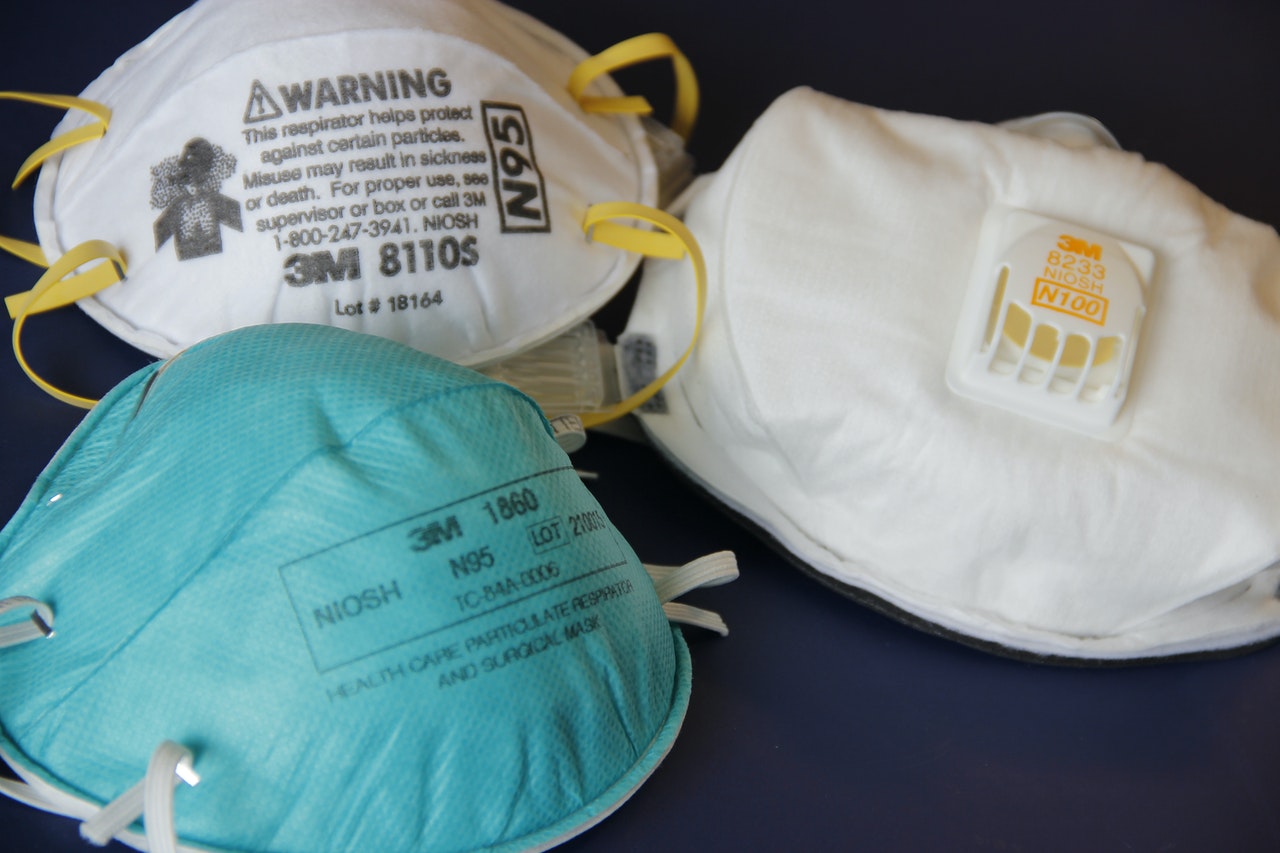
The Trump administration has eased a ban on exports of PPE, with the list of exemptions growing after lawyers laid out the shortcomings of the rules.
Exports to Canada, Mexico and U.S. entities such as military bases abroad are among the exemptions. It also singles out shipments by 3M, which President Trump had originally blocked from sending N95 masks to Canada and Latin America. The decision was reversed last week after reaching a production agreement with the company.
The previous rule overlooked instances where multinational firms might need to ship PPE to overseas offices or military bases, showcasing the dangers of regulating trade in vital medical supplies unilaterally by anecdote, rather than after careful review and consultations with key trading partners.
The focus of the ban is on “commercial quantities,” which is defined as shipments valued at US$2,500 and containing more than 10,000 units of gloves, masks and other covered materials.
As this issue continues to evolve, please do not hesitate to reach out to Carson for guidance around PPE shipments at this time.
Get in touch:
Tyler Carson, President, tyler@carson.ca
Dave Pentland, VP, dwpentland@carson.ca
Matt Earish, COO, matt@carson.ca